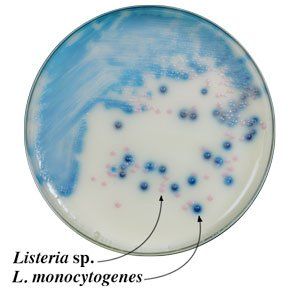
1. Highly differential medium that works by the combination of indoxyl derivative chromogenic substrates that produce positive color reactions for colonies of nonpathogenic Listeria sp. that are pink due to their ß-glucosidase activity, and blue-green to blue-violet for the pathogenic species depending on the strain-specific balance of ß-glucosidase (pink) and phosphatidylinositol-specificphospholipase C (blue) activities.
2. Other companies’ selective media for Listeria species that only depend on the detection of ß-glucosidase activity produce a single color for colonies for all Listeria species.
3. The agar surface of our plates have an opaque white background that facilitates differentiation of colored colonies of both groups of Listeria organisms growing on the surface of the plate.
4. Our medium’s selectivity and sensitivity for the pathogenic Listeria species (L.m. & L.i.) is 96% and 100%, respectively, with no false negatives.
5. Water insoluble chromogenic lattice prevents the colony color from diffusing into the agar making detection and enumeration much easier.
6. Competitors’ non-chromogenic agars (MOX, Oxoid & Palcam) which depend on esculinase as the differential trait allows many Listeria sp., Bacillus sp. and Enterococcus sp. to grow as black color colonies. Since the end product is water soluble, the black color will diffuse into the medium. Additional expensive tests are required to further identify L.m. strains.
7. Competitors’ chromogenic media which utilize the X-β-D-glucopyranoside substrate will allow too many Listeria sp., Bacillus sp. and Enterococcus sp. to grow as grow as blue colored colonies without clear halos. These colonies will interfere with the identification of L.m. & L.i. by coalescing together making it difficult to see the clear halo around the blue colony of the L.m. & L.i.
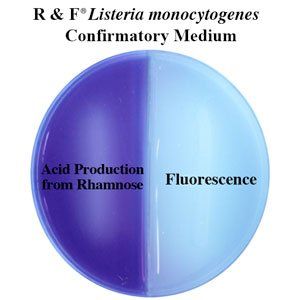
8. Our rapid and convenient fluorogenic/acid test differentiates L.m. from L.i. as quick as 6 hours-saving time and costs.
9. The shelf life of prepared plates remains stable for at least 60 days, stored in the dark at 2-8°C. One bottle of plating medium powder and two boxes of supplements will make approximately 230 plates.